
Uppsala Reports editor
@UMCGlobalSafety

Since 2021 PAHO has assisted 12 countries in importing more than 270,000 historic and new cases to the global database.
Pharmacovigilance relies on information systems to extract data to conduct appropriate medicines safety surveillance. These systems, which must be robust, efficient, and standardised, are necessary to help detect and assess signals to mitigate risks of medicinal harm. The COVID-19 pandemic, however, highlighted the limitations of such systems to collect quality information on adverse events following immunisation (AEFIs), particularly during the initial deployment of the COVID-19 vaccines. Many countries relied on outdated methods like paper forms and spreadsheets, which lacked standardised terminology and harmonised formats. Considering the urgency to report adverse events and the limitations regarding traditional reporting methods, the pandemic acted as a powerful catalyst for national pharmacovigilance systems, particularly the National Regulatory Authorities (NRAs), to modernise and strengthen their information systems.
Recognising the growing challenges and needs in the region, the Quality and Regulation Unit of the Pan American Health Organization (PAHO), with support from the Government of Canada, launched the “Data Bridges” project in 2021. This initiative aimed to strengthen the quality of transmission of individual case study reports between countries across the region and VigiBase through customised digital tools and applications. As a result of this collaborative effort, NRAs enhanced their abilities to access, exchange, and integrate pharmacovigilance data in a cohesive, coordinated way, contributing to strengthening safety surveillance at local, national, regional, and global levels.
Since its inception, the Data Bridges project has been implemented in 12 countries in Latin America and the Caribbean in close collaboration with NRAs, immunisation programs, PAHO, and Uppsala Monitoring Centre (UMC) in a unified effort to improve data processing and reporting to VigiBase. These stakeholders play distinct but crucial roles in data transmission (see Figure 1). NRA involvement has been vital for understanding data, mapping databases to the E2B format (ICH standard required by VigiBase), and validating results. Meanwhile, UMC supports data import into the global database, provides technical feedback and assistance in drug standardisation, and ensures E2B compliance.
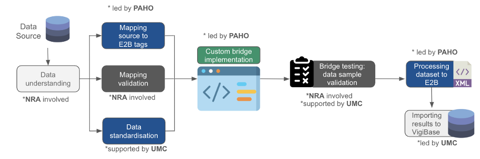
Through this partnership, Data Bridges has enabled the successful processing and transmission of 97,433 COVID-19-related AEFI from countries including Paraguay, Mexico, Peru, Chile, Argentina, and Belize to the global database. Beyond COVID-19, the project has also facilitated the transfer of 180,940 records of adverse drug reactions (ADRs) and non-COVID-related AEFIs from Brazil, Peru, Colombia, Cuba, Costa Rica, and Chile.
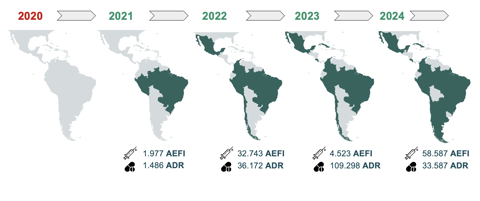
The project’s success highlights the transformative power of digital infrastructure in pharmacovigilance. As PAHO continues to support Brazil, Mexico, Chile, Paraguay, and Uruguay, future efforts will focus on enhancing continuous ADR and AEFI data transmission between NRAs and VigiBase. Plans are also underway to further support new NRAs in adopting technologies for more efficient integration of drugs and adverse events into VigiBase, ensuring that digital transformation in pharmacovigilance keeps pace with emerging challenges and contributes to public health across the Americas.
As those working in public health and pharmacovigilance continue to face the likelihood of new pandemics and global health crises, initiatives such as the PAHO project are imperative to support NRAs in their pharmacovigilance processes as they work to ensure the public’s safety during these times.
This article was written in collaboration with PAHO project members Ana María Reyes, José Luis Bustos, María José Alfonso, and Robin Rojas Cortés. If you would like to know more about the project, contact rojasedg@paho.org.
WHO and UMC introduce new VigiBase access conditions, enhancing data sharing clarity and stakeholder confidence in global pharmacovigilance.
20 March 2025
The theme of this year’s WHO PIDM programme members meeting investigated the true value of social media in signal detection and creating effective communication strategies.
19 October 2023
Since its introduction 20 years ago, UMC’s adverse event reporting system has become the world’s most widely used safety surveillance toolkit for medicines and vaccines.
25 November 2024