
Jose Antonio Maza Larrea
Physician | Chemist, Pharmacist and Biologist (QFB)

Photo: iStock
Undaunted by the pandemic, the team at Instituto Nacional de Cardiología Ignacio Chávez (Mexico) established a PV centre and a follow-up unit with direct, daily clinical impact.
A the Instituto Nacional de Cardiología Ignacio Chávez (INC) in Mexico City, we have developed a pharmacovigilance system with a clinical focus, looking forward to making medication safer by identifying, evaluating, and reporting the adverse drug reactions (ADR) that patients develop in the institute.
To constitute this project, we joined efforts with various people in the institute to manage the formalities, manuals, procedures, and documentation; secure the sufficient human and material resources; and ensure compliance with the relevant legal requirements in the country. This work culminated in December 2020, with the establishment of the Clinical Pharmacology Department, comprising the Institutional Centre of Pharmacovigilance (ICPV) and Pharmacotherapeutic Follow-up Unit (PFU).
Our work entailed developing a whole pharmacovigilance system, starting by registering the ICPV with the health authority at the National Centre of Pharmacovigilance at the Comisión Federal para la Protección contra Riesgos Santiarios (COFEPRIS) and establishing the objectives, mission, and vision of this new department.
Provide pharmaceutical care to patients in the institute, promoting a pharmacovigilance notification culture and evaluating, comprehending, reporting, and preventing adverse drug reactions.
To be a national and international reference in pharmacovigilance science focused on cardiovascular treatment, delivering risk-benefit actualised information related to drugs, and making medication safer.
We set up the department logo to be the base of all communications around pharmacovigilance in our Institute.

For the operation of the ICPV, we developed a manual, establishing the roles of all pharmacovigilance actors and the standard operating procedures. In particular, the collaboration with the PFU is of singular importance to consolidate the pharmacovigilance system, as is the role of the clinical pharmacists working with clinicians and patients to identify ADRs.
This clinical-based system is targeted to make pharmacovigilance more accessible to clinicians, helping them to make wiser therapeutical decisions. As former UMC director, Dr Marie Lindquist once said, “We are here to improve the health and wellbeing of patients taking medicines, wherever they are”.
To accomplish this, we have a team of pharmacists that every day analyse the pharmacotherapeutic treatment of the patients, identifying medication errors, and making pharmaceutical interventions to reduce risks by making suggestions to the clinicians to improve their prescriptions. With this method, the prescriptions of the hospitalised patients were reviewed and the PFU team made 2521 interventions, reducing the risk of prescription errors by 45.4% in 2021.
Today we have an operating ICPV that day by day secures better procedures and identifies ADRs, evaluating and reporting them, as well as giving feedback to the prescriber in the clinical environment – all contributing to make medication safer.
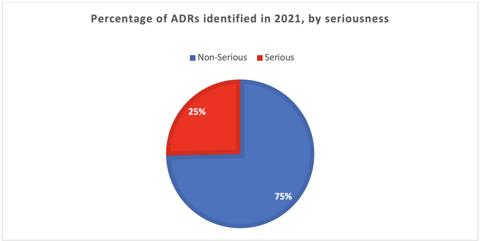
We identified, evaluated, and reported 146 ADRs throughout 2021 (see the chart below). It is important to declare that at the beginning of the year, we had an active pharmacovigilance study and, therefore, identified more ADRs. Overall, 75% of the ADRs identified were non-serious.
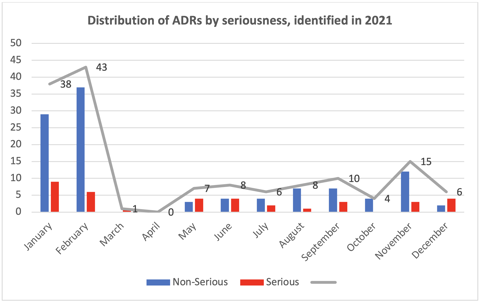
These reports do not include adverse events following immunization (AEFI), which were evaluated separately from the ADRs. In all, we identified, evaluated, and reported 331 AEFIs related to administration of COVID-19 and influenza vaccines.
In conclusion, even in the midst of the COVID-19 pandemic, our team was able to establish an effective pharmacovigilance centre and a follow-up unit, both of them with a direct clinical impact on improving patient treatment and medication safety by becoming an essential part of the decision-making process in day-to-day patient treatment.
With additional contributions from Mtra. Franchesca Rosado Hernández, Mtra. Ingrid Gutiérrez Villegas and QFB. Susana Fernández Rosas
Two-thirds of pharmacists in Nigeria witness weekly cough syrup abuse, yet poor reporting systems and unclear guidelines prevent effective intervention, leaving its youth at risk.
03 December 2025
Since 2020, the ICPV has worked to improve drug safety reporting at Instituto Nacional de Cardiología Ignacio Chávez, with promising outcomes.
16 October 2025
The annual #MedSafetyWeek campaign reminds us that medicines work best when they're safe. In Aligarh, healthcare workers came together to make that message a living reality.
19 November 2025