
Arshia Bhandari
Pharmacovigilance consultant & medical writer

Effective risk communication is a mainstay of successful pharmacovigilance, yet examples of poor risk mitigation abound. Where are we failing, and what can be done about it?
Despite years of experience with “smoking is injurious to health” disclaimers and graphic warning labels on cigarette packaging, existing research does not paint a black and white picture of their effectiveness. A study in 2019 found that “graphic health warnings on cigarette packs did not influence participants' purchase of cigarettes as a main effect. Though it reduced the chances of cigarette purchases for smokers lower in nicotine dependence, it had no influence on smokers higher in dependence”. Likewise, an Australian study investigating the effectiveness of pictorial warnings in Australia showed that “the policy only led to a short-term increase in the probability to quit smoking and mildly reduced the probability to relapse in the year of the reform. The results for the short-term effects on quitting were consistent with the previous literature that has documented a wearing-out effect”.
To understand the consumer’s perspective on this issue, a short LinkedIn survey by the author on the effectiveness of graphic warnings in deterring people from smoking showed 73% of 81 participants believed it to have no effect.
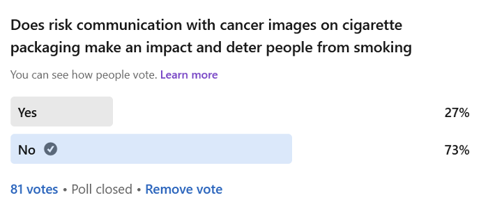
Why are the apparent results of such clear risk communication so mixed?
Take another example, risk communication regarding valproate, a drug prescribed for epilepsy and bipolar disorder that poses risks when taken during pregnancy. According to a review conducted by the European Medicines Agency (EMA) in 2014 on the drug, “two in five babies exposed to valproate in the womb develop cognitive problems and one in 10 babies are at a risk of developing congenital malformations.” The scale of the issue and harm caused is considered bigger than thalidomide. In 2018, the pharmacovigilance risk assessment committee recommended new measures to avoid foetal valproate exposure.
To measure the impact of the 2018 European label changes and the newly developed pregnancy prevention programme for medicinal products containing valproate, the European Medicines Agency (EMA) commissioned a drug utilisation study which was published in June 2023. The study showed the new measures had a “small impact” on valproate utilisation and prescription in the studied European countries. Further, the substantial number of concurrent pregnancy events with valproate exposure warranted careful monitoring of the existing pregnancy prevention programme for valproate in clinical practice in Europe, to see if there is a need for additional measures in the future.
Eudravigilance data for valproate from 2020 to 2023 shows 200+ foetal exposure cases with congenital anomalies in the 0-2 years age group. Despite multiple alerts, label updates, constant warnings to physicians and patients, and pregnancy prevention programmes, valproic acid continues to cause harm. Where could we be failing?
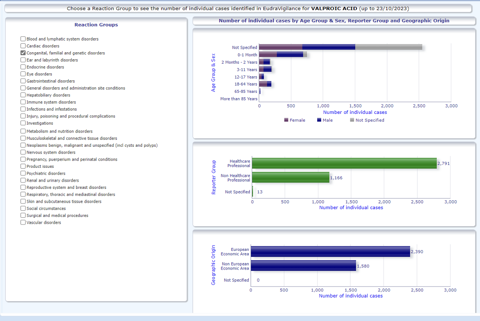
The effectiveness of risk communications is determined by a number of factors. For example, in 2013, an exclusive report by the BBC showed that in Brazil, the drug thalidomide was still being used during pregnancy, despite the known risk that it causes birth defects. The drug is prescribed to leprosy patients for symptomatic relief, and pregnancy prevention forms are usually signed during prescription. However, some women had been taking it during pregnancy unaware of the teratogenic risks of Thalidomide. The issue was observed in patients staying in more remote areas, where health illiteracy is more prominent, and geographic isolation may impact the reach of risk communications to those areas. Eudravigilance data still shows one case of phocomelia in an infant reported in October 2023 associated with Thalidomide exposure during pregnancy in the non-European economic area.
Various other studies that have investigated why risk communications may fail have found other factors at play. Some of these factors include:
In the case of smoking, behavioural factors, communication fatigue, and ambiguity in messaging due to endorsing tobacco products by influencers could be the reasons why risk communication in this field have a limited impact. As for valproate, surveys published by epilepsy charities have shown that one in five women taking valproate remain unaware of the potential severe risks of its use in pregnancy despite valproate risk communications (e.g., Medicines and Healthcare products Regulatory Agency tool kits).
A 2023 systematic review investigated factors influencing the implementation of medicine risk communications by healthcare professionals into clinical practice. They identified a “complex interaction between stakeholders (health authorities, pharmaceutical companies, healthcare professionals, patients, and their carers) involved in the creation and implementation of risk alerts” as a factor impacting their efficacy.
In an interview with Dr. Pipasha Biswas, ex-Drug Safety Research Unit (DSRU) Clinical Research Fellow and senior consultant in Pharmacovigilance, she emphasises the importance of constant interactive and effective communication with the health care professionals and patients to integrate risk mitigation through pharmacovigilance in clinical practice. As a DSRU fellow, she was involved with Prescription Event Monitoring studies for newly authorised products in the UK including psychiatric drugs. Her work with DSRU gave her a chance to regularly interact with general practitioners through timely follow-up including targeted questionnaires. She observed that “the information through ‘Dear Doctor’ letters, literature, or regulatory newsletters could get lost in a physician’s office due to paucity of time, other emergency matters, or lack of interest. For risk communications to have effect, a two-way constant interaction and targeted communication to the right audience is important”. She further adds that considering the importance of communication in pharmacovigilance, it could be prudent to amend the definition of pharmacovigilance to the pharmaceutical science relating to the "collection, detection, assessment, monitoring, prevention, and effective communication" of adverse effects with pharmaceutical products.
Dr. Biswajit Chakrabarti, a consultant physician at Liverpool University Hospitals NHS Foundation Trust, sees technology as a game changer to make risk communications more effective. He observes that “healthcare professionals are bombarded year upon year with an increasing number of complex clinical guidelines and alerts from multiple specialists in the hope that what is contained in these guidelines will somehow be applied to improve patient care in daily clinical practice. Despite the worldwide availability of evidence-based guidelines to healthcare professionals, the level of effective guideline implementation is hugely variable. For example, the key themes emerging from national asthma audits in the UK (since 1963) have remained unchanged over time with little evidence of improvement in care and outcomes, despite widespread access to clinical guidelines. Introduction of clinical decision support system software within patient pathways may help to address this challenge, with key clinical guidelines embedded intelligently within its algorithms thus guiding the physician from symptoms to diagnosis and then intelligently prompting guideline-level management”.
The clinical decision support systems could also be configured to ease documentation and reporting of adverse events. Generative AI tools are still in their infancy, and many concerns linger about their accuracy and reliability, but they nevertheless show some promise for being able to help with effective dissemination, access, and understanding of risk communications.
Last but not least, it is important to evaluate the effectiveness of risk communication regularly and make appropriate changes based on regular feedback.
From a physician’s lens, whose focus is treating the patient, sometimes the risks of a drug can be undermined due to myriad factors. However, correct, consistent messaging given at an optimum frequency, accessibility, choice of the right audience, and integration of technology for risk communications could contribute positively towards making it effective in pharmacovigilance, whether for adverse drug reaction management or reporting.
Disclaimer: The views expressed in this article are those of the individual authors writing in their individual capacities only and do not necessarily reflect the official policy or position of their respective employers. Responsibility for the information and views expressed in Uppsala Reports articles lies entirely with the authors. Articles do not necessarily reflect the policies or positions of Uppsala Monitoring Centre.
Over 500 attendees from 80+ countries met in Cairo at ISoP 2025, reflecting on pharmacovigilance progress and shaping strategies for its future.
10 December 2025
Medical devices are everywhere, but monitoring their safety is complex. Omar Aimer discusses the unique challenges of device pharmacovigilance and what needs to change.
29 January 2026
Patient engagement was a hot topic at the fireside chat held during this year’s ISoP mid-year symposium.
24 June 2025