
Paul Brewer
SF Global Communications Programme Lead, Medicines & Healthcare products Regulatory Agency (MHRA), UK

Substandard in vitro diagnostic kits for malaria found in Tanzania.
WHO is taking the fight to falsified and substandard medicines with a coordinated global communication strategy, to combat the growing threat to public health from these products.
It is essential that healthcare professionals understand and are able to protect themselves and their patients from the risks associated with substandard and falsified (SF) medical products. Falsified medicines, in particular, may contain no active ingredient, the wrong active ingredient, or the wrong amount of the correct active ingredient. They are also found to commonly contain corn starch, potato starch, or chalk. Some SF medical products are toxic in nature, with either fatal levels of the wrong active ingredient or other toxic chemicals. They are often produced in unhygienic conditions by unqualified personnel and contain unknown impu-rities, and they are sometimes contaminated with bacteria.
SF medical products are, by their nature, difficult to detect. They are often designed to appear identical to the genuine product and may not cause an obvious adverse reaction. However, they will often fail to properly treat the disease or condition for which they were intended and can lead to serious health consequences, including death.
What was once considered a problem limited to low- and middle-income countries now affects all countries. With the exponen-tial increase in internet connectivity, those engaged in the manufacture, distribution and supply of SF medical products have gained access to a global marketplace. A culture of self-diagnosis and self-prescribing has led to the emergence of thousands of unregulated websites providing unsupervised access to SF medical products. However, according to WHO, it is low- and middle-income countries and those in areas of conflict or civil unrest, where health systems are weak or non-existent, that still bear the greatest burden of SF medical products.
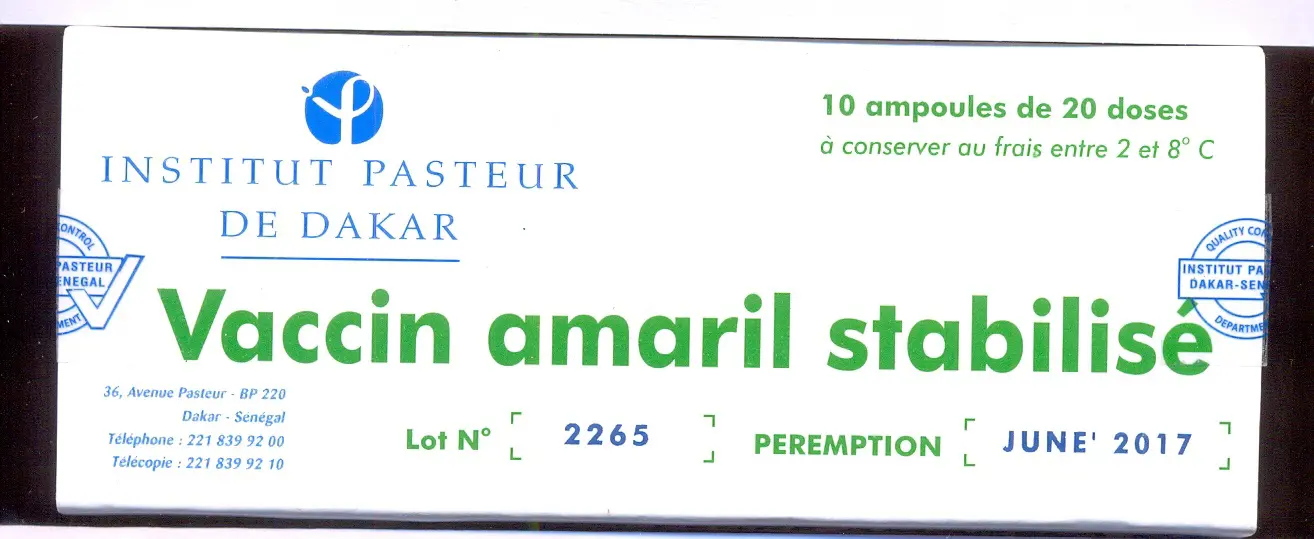
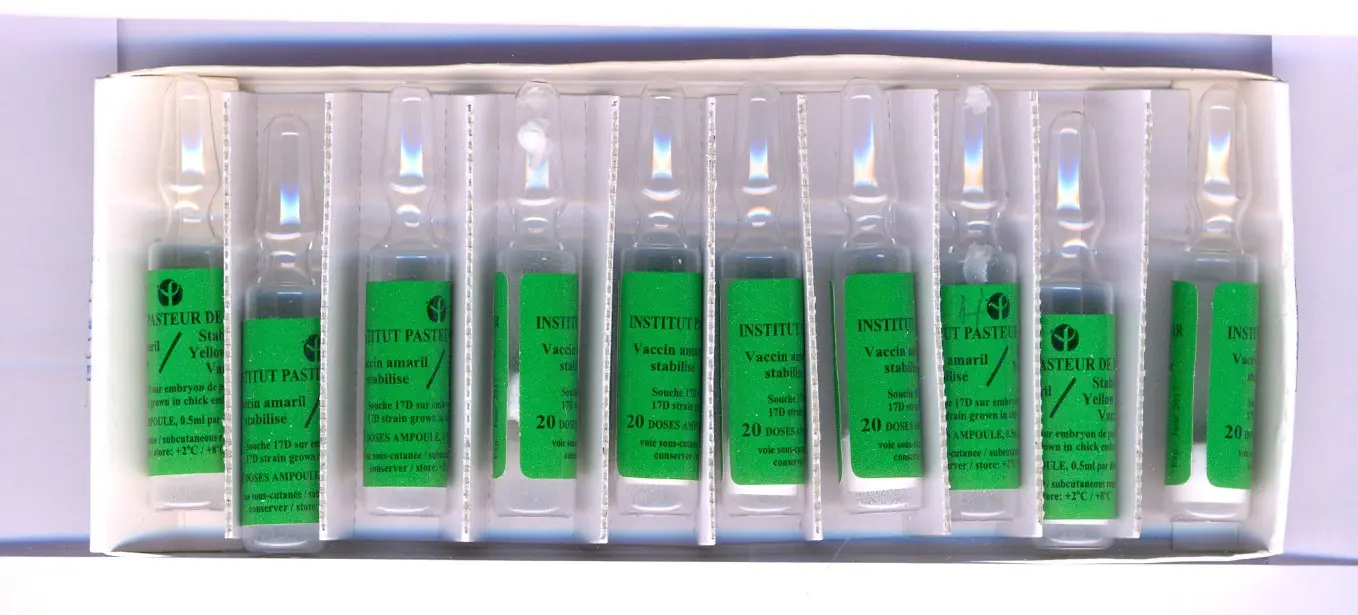


A 2017 study by WHO estimated that one in 10 medical products in low- and middle-income countries is substandard or falsified. The study was based on more than 100 published research papers on medicine quality surveys undertaken in 88 low- and middle-income countries involving 48,000 samples of medicines. Lack of accurate data means that these estimates are just an indication of the scale of the problem. As a result, in 2012 WHO established the Member State Mechanism, a global platform to tackle SF medical products. In 2013, WHO launched the Global Surveillance and Monitoring System (GSMS) and has now trained a worldwide network of over 700 regulatory personnel in 150 member states to encourage reporting of incidents involving SF medical products, and to help develop a more accurate and substantiated assessment of the problem.
The GSMS provides technical support in emergencies, links incidents between countries and regions, and issues WHO global medical product alerts. It also gathers evidence to more accurately demonstrate the scope, scale, and harm caused by SF medicines, and to identify vulnerabilities, weaknesses, and trends. Since its launch, WHO has received 2,000 reports of SF products from around the world. Medicines from all major therapeutic categories have been reported, including vaccines and diagnostics.
Underpinning the work of the member states is a comprehensive and integrated strategic approach with interventions at all stages in the journey of medicines to patients, summed up as: prevention, detection, and response. One of the work streams aims to address the prevention element of the strategy by giving member states a framework and tools to develop communications campaigns to alert patients and healthcare professionals to the risk of SF medicines. Work has recently been completed to provide detailed guidance in the form of a communications handbook, alongside a digital library of campaign materials to enable all WHO member states to accelerate campaign planning, implementation, and measurement in their efforts to combat SF medicines.
Substandard (also called "out of specification") are authorised medical products that fail to meet quality standards or specifications, or both.
Unregistered/unlicensed medical products have not undergone evaluation and/or approval by the national or regional regulatory authority for the market in which they are distributed or used.
Falsified medicines deliberately and/or fraudulently misrepresent their identity, composition or source.
Read more:
How can we make healthcare safer for children? ISoP’s Angela Caro-Rojas shares insights ahead of World Patient Safety Day.
04 September 2025
Oman’s VigiMobile launch is more than a technical upgrade. It signals a strong commitment to patient safety and public participation in pharmacovigilance.
17 September 2025
Prof. Singhal passed away on 19 April 2025. He is survived by his wife, children, and a large community of pharmacology researchers who had the privilege of training under him.
02 July 2025