
In 2018, the Saudi Food and Drug Authority (SFDA) launched a strategic project for regional pharmacovigilance centres, to enhance pharmacovigilance activities among health institutions across the Kingdom of Saudi Arabia. The project aligns with SFDA’s responsibilities, which include the ongoing monitoring of the safety, efficacy and quality of pharmaceutical products in the kingdom.
Saudi Arabia currently has 25 regional pharmacovigilance centres geographically distributed to maintain continuous pharmacovigilance activities in all regions. Their duties and responsibilities include reporting adverse drug events (ADEs) to the National Pharmacovigilance Centre (NPC); responding promptly in case of crises (such as the current COVID-19 pandemic); performing awareness campaigns and workshops; and following-up on ADE reports.

“This strategic project has had a positive impact in ensuring medication safety through active communication and connecting all reference hospitals together to improve pharmacovigilance activities in the Kingdom,” says Adel Al-Harf, Vice President for Drug Sector at SFDA.
Ali Al-Shahrani, Executive Director of Pharmacovigilance at SFDA, also emphasises the importance of creating collaborative communities.

“Regulatory body communication with healthcare providers about pharmacovigilance is crucial,” Al-Shahrani says. “Therefore, this project aimed to build an effective pharmacovigilance community across hospitals in the Kingdom to improve communication about and understanding of pharmacovigilance activities and delegate some tasks to regional centres to utilise human resources properly.”
As noted above, NPC is responsible for collecting and evaluating ADE reports in the kingdom, as well as identifying signals of adverse drug reactions (ADR) and promoting a reporting culture. Therefore, NPC has taken several initiatives with a number of regional pharmacovigilance centres to enhance patient safety, which included workshops, educational material, and motivational events.
The project's first milestone was in February 2019, with training visits to all regional centres, covering topics, such as NPC responsibility, an overview of pharmacovigilance, and the regional pharmacovigilance centres’ objectives.
Raising awareness among the public and healthcare providers about pharmacovigilance is an important role of the regional pharmacovigilance centres, and in support of that, NPC provided educational materials to each centre to display in their organisation and hand out to their patients. These educational materials included roll-up banners, posters, and ADE patient reporting cards.
In addition, to boosting motivation, an appreciation event was held on World Patient Safety Day. The event aimed to introduce the audience to the regional centres’ integral role in monitoring pharmaceutical products’ post-marketing safety throughout the Kingdom, and it was also an opportunity to present awards to organisations and honour their achievement.
Overall, this strategic project has had a positive effect on both the quantity and quality of reporting. The number of ADE reports received from health institutions at NPC gradually increased after the project started. In particular, the regional centres reported 28,021 cases, representing 67% of all health institutions reports (41,603 reports) in 2020. Just as importantly, the quality of ADE reports received from healthcare providers improved significantly after the establishment of regional pharmacovigilance centres in terms of completeness, comprehensiveness, and response to follow-ups. This improvement was attributed to the educational efforts done by the regional centres’ representatives, which aimed to enhance the reporting culture among the staff of their corresponding institutions.
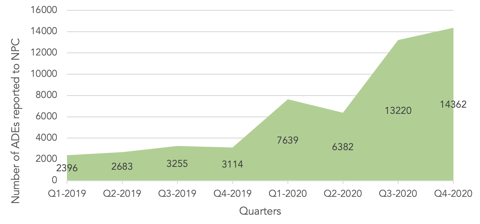
Regional pharmacovigilance centres are relatively new to the Kingdom; however, their contribution has already made a significant improvement in the reporting culture, as evidenced by the increases in ADE reporting. Plans for future developments include further promotion of awareness regarding pharmacovigilance activities and more enhancements to the reporting culture and quality of reporting.




