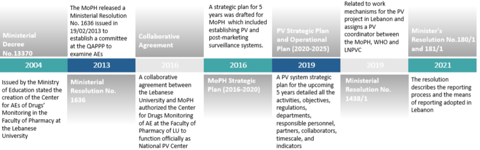
The infrastructure was defined by outlining all the stakeholders involved: policymakers, regulatory authorities, the pharmacovigilance operating entities, and the pharmacovigilance reporting entities. Once the legal framework, infrastructure and human resources were in place, the QAPPP developed the means for reporting adverse events.
Patients, healthcare professionals and marketing authorisation holders (MAHs) can send reports either through the national adverse events reporting form, a landline hotline, or an e-reporting service. Reports received are coded, analysed and assessed at the LNPVP using VigiFlow, an electronic pharmacovigilance management database, and sent to the WHO global database VigiBase. Staff members have been trained in data collection, and cleaning and coding of adverse events using MedDRA (Medical Dictionary for Regulatory Activities) terminology. Since active participation is vital from all stakeholders, HCPs and MAHs were made aware of the importance of reporting. In turn, the trained HCPs were responsible for educating patients on COVID-19-related AEFI, thus closing the loop with all relevant contributors to the post-marketing surveillance programme.
As a result of these initiatives, the LNPVP started receiving ICSRs, and thus became a full member of the WHO PIDM in 2021. Reporting during the COVID-19 mass-vaccination campaign greatly enhanced the system. The introduction of new reporting tools such as a national COVID-19 vaccine hotline (1214 Hotline), a vaccination module in the e-Governance platform in Lebanon, and an open-source toolkit for data collection and analysis in humanitarian emergencies (KoBotoolbox), boosted reporting from 20 to 100 weekly AEFI case reports. The effective screening of the AEFI reports resulted in a better classification and thus improved management of AEFI. Field visits to vaccination centres ensured compliance with operating standards, and a monthly report summarising AEFI findings was circulated.