
SITUATED AT THE EASTERN END of the Mediterranean Sea, Lebanon has been making sound steps forward in medicines safety in recent years. Home to over six million people (although there is a large Lebanese diaspora, in the Americas especially), the official language of the country is Arabic, while French is formally recognised. Vast forests of cedar trees once covered much of Lebanon, hence the national emblem of the country and the centrepiece of its flag.
The establishment of a pharmacovigilance system is particularly difficult in resource-limited countries such as Lebanon. After some preliminary activity, and having become an associate member of the WHO Programme for International Drug Monitoring (PIDM) in 2018, pharmacovigilance activities, as core functions of the Ministry of Public Health (MoPH), were moving forward when the COVID-19 pandemic began. The implementation of an effective national pharmacovigilance system in Lebanon should be seen in the context of the effects of COVID-19.
The route to that success was initiated by establishing the legal groundwork with technical assistance from WHO offices. The Quality Assurance of Pharmaceutical Products Program (QAPPP) issued a series of regulations that facilitated the system’s organisation and functions, to further strengthen the framework for pharmacovigilance, set out in the graphic below.
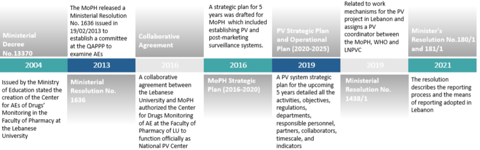
Under the supervision of the QAPPP within the Lebanese MoPH, the Lebanese National Pharmacovigilance Program (LNPVP) was created, and the national centre was admitted as a full member of the WHO PIDM in February 2021 as shown in the timeline below.

THE INFRASTRUCTURE WAS DEFINED by outlining all the stakeholders involved: policymakers, regulatory authorities, the pharmacovigilance operating entities, and the pharmacovigilance reporting entities. Once the legal framework, infrastructure and human resources were in place, the QAPPP developed the means for reporting adverse events.
Patients, healthcare professionals and marketing authorisation holders (MAHs) can send reports either through the national adverse events reporting form, a landline hotline, or an e-reporting service. Reports received are coded, analysed and assessed at the LNPVP using VigiFlow, an electronic pharmacovigilance management database, and sent to the WHO global database VigiBase. Staff members have been trained in data collection, and cleaning and coding of adverse events using MedDRA (Medical Dictionary for Regulatory Activities) terminology. Since active participation is vital from all stakeholders, HCPs and MAHs were made aware of the importance of reporting. In turn, the trained HCPs were responsible for educating patients on COVID-19-related AEFI, thus closing the loop with all relevant contributors to the post-marketing surveillance programme.
AS A RESULT OF THESE INITIATIVES, the LNPVP started receiving ICSRs, and thus became a full member of the WHO PIDM in 2021. Reporting during the COVID-19 mass-vaccination campaign greatly enhanced the system. The introduction of new reporting tools such as a national COVID-19 vaccine hotline (1214 Hotline), a vaccination module in the e-Governance platform in Lebanon, and an open-source toolkit for data collection and analysis in humanitarian emergencies (KoBotoolbox), boosted reporting from 20 to 100 weekly AEFI case reports. The effective screening of the AEFI reports resulted in a better classification and thus improved management of AEFI. Field visits to vaccination centres ensured compliance with operating standards, and a monthly report summarising AEFI findings was circulated.
LNPVP PERFORMED ADDITIONAL STEPS to strengthen the AEFI surveillance mechanism, via signal detection and validation to identify signals associated with the Pfizer-BioNTech and AstraZeneca COVID-19 vaccines. This was hindered at many levels by the shortage of staff and lack of sustainable financial support. The response to COVID-19 coincided with a turbulent political and economic situation disrupting all of society including health. Yet, despite these financial, political and technical difficulties the Lebanese pharmacovigilance programme established a rigorous safety profile, and in a short period received 7,000 case reports and 24,837 AEFI, following the administration of 5,134,093 doses of COVID-19 vaccines.

CURRENTLY, THE PHARMACOVIGILANCE TEAM at the QAPPP is working on guidelines on good pharmacovigilance practice for MAHs. After strengthening the passive reporting system, the LNPVP is planning to establish an active surveillance system. Finally, with the vision of keeping the LNPVP up-to-date with technological advancements, the programme has recently adopted the safety app developed by MHRA as a new smart reporting tool. Pharmacovigilance is sending out strong branches across Lebanon for an evergreen future.
See also
H Abbas, A Zeitoun, M Watfa, et al, “Implementation of a Pharmacovigilance System in a Resources-Limited Country in the Context of COVID-19: Lebanon’s Success Story”, Therapeutic Innovation & Regulatory Science, 2022.




