
Orphan drugs, which are medicines developed to treat rare conditions, often have limited exposure before their market release due to the small number of patients they target. As a result, these medicines lack comprehensive safety data, which is essential to identify and evaluate potential adverse effects. Thus, post-marketing safety monitoring of orphan drugs is crucial in pharmacovigilance to enable regulatory bodies, healthcare professionals, and patients to make informed decisions regarding their use.
To this end, the Saudi Food and Drug Authority (SFDA) initiated a project aimed at detecting, managing, and monitoring safety concerns related to registered orphan drugs to improve the safety profiles of these drugs and optimise patient care.
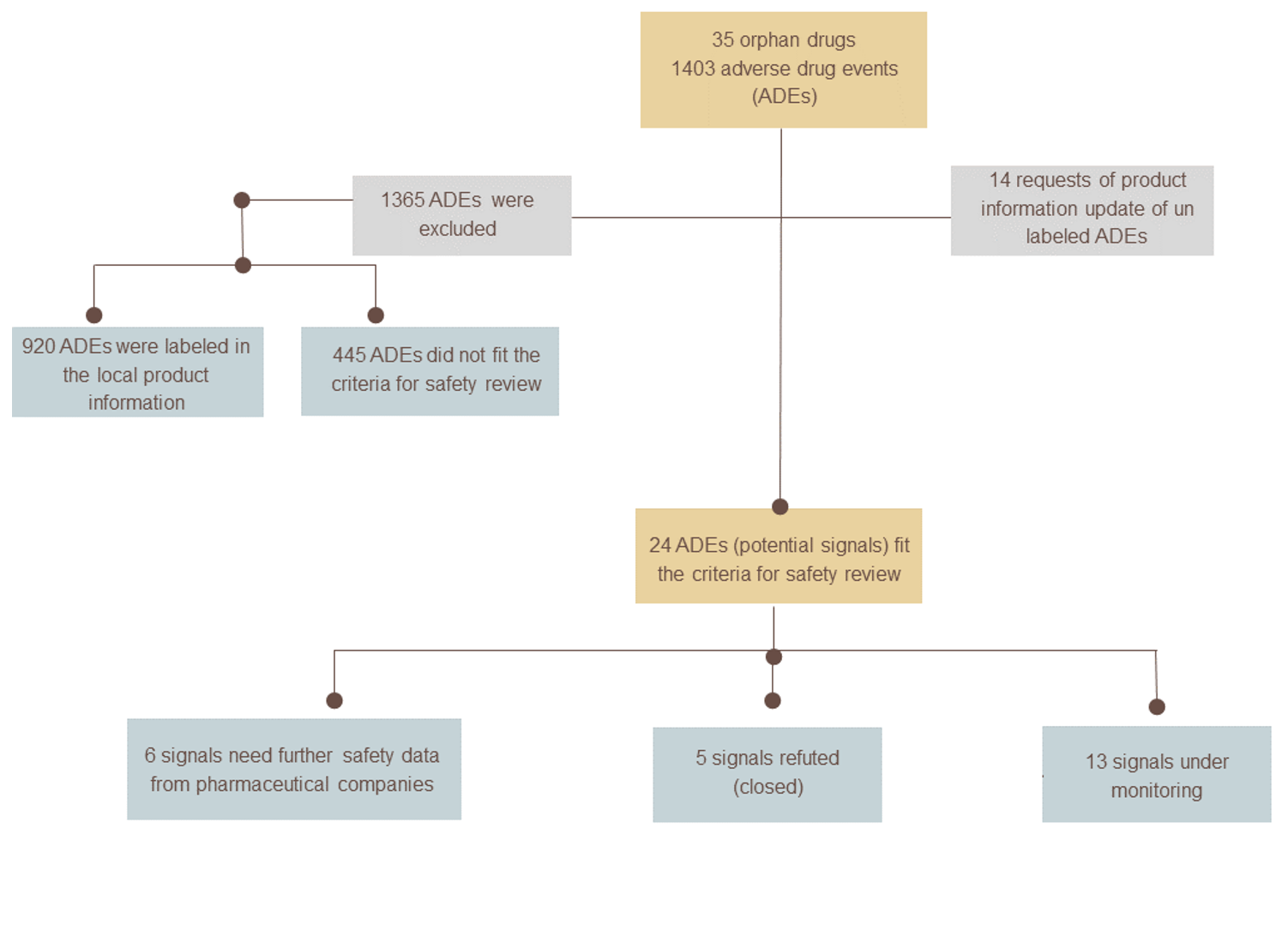
The project followed a systematic approach, whereby a list of SFDA-registered orphan drugs from 2016 to 2023 was thoroughly investigated. For each drug in the list, published reports of adverse events were retrieved from the AdisInsight database, and a thorough assessment was conducted to exclude labelled adverse events. A comprehensive review of drug safety was then conducted for the remaining adverse events that were serious, and for which there was evidence from literature available. The review utilised various sources of evidence, including unpublished clinical trials, published scientific literature, local and global spontaneous reports, and periodic benefit-risk evaluation reports.
The project comprised the evaluation of a total of 35 orphan medications registered by the SFDA, revealing 1403 reported adverse events linked to these medications retrieved from the AdisInsight database. As a result of the assessment process, requests have been made to update the local product information of 14 orphan drugs from pharmaceutical companies. Additionally, we performed 20 comprehensive drug safety reviews to assess 24 potential safety signals. The recommendations stemming from these reviews included requests for further safety data for six signals, continued monitoring of 13 signals, and the discontinuation of monitoring five other signals.
In addition to the safety monitoring of orphan drugs project, the SFDA conducted a workshop entitled "Orphan Drugs: SFDA's Initiatives and International Experience in Orphan Drug Regulations and Post-marketing Safety of Orphan Drugs". This workshop aimed to raise awareness of orphan drug regulations and post-marketing safety practices in Saudi Arabia and the rest of the world.
The workshop garnered significant attention, with a total of 127 attendees from various backgrounds and expertise. It was conducted in two sessions, the first serving as an introduction to orphan drugs, with an overview of the orphan drug landscape in Saudi Arabia. The overview focused on the regulatory frameworks, challenges, and achievements in the local context.
The second session focused on orphan drug regulations and post-marketing safety at the international level. Expert speakers discussed safety-related regulatory actions for orphan drugs in the US and EU, presenting findings from a cohort study. The experiences of the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) were also discussed, emphasising their collaboration in the Experience Cluster on Rare Diseases and Orphan Medicinal Products project. The workshop provided valuable insights and highlighted the importance of continuous effort in monitoring the safety of orphan medications.
Overall, the SFDA safety monitoring of orphan drugs project has made significant strides in enhancing the identification and evaluation of potential safety signals linked to the use of orphan drugs, despite the obstacles presented by limited evidence and reported cases. Raising awareness about the reporting of adverse drug events, particularly in the context of orphan drugs, is of paramount importance to ensure the continued safety of patients regardless of disease rarity.
Read More
“EMA Cluster activities”, European Medicines Agency, 2008.
Listen up
"The challenge of rare diseases – Christina Ström Möller", Drug Safety Matters podcast







