
Jose Antonio Maza Larrea
Physician | Chemist, Pharmacist and Biologist (QFB)

Since 2020, the ICPV has worked to improve drug safety reporting at Instituto Nacional de Cardiología Ignacio Chávez, with promising outcomes.
When the Institutional Centre of Pharmacovigilance (ICPV) and a Pharmacotherapeutic Follow-up Unit (PFU) at Mexico's Instituto Nacional de Cardiología Ignacio Chávez were established in 2020, their primary goal was responding to the COVID-19 pandemic. What began as an emergency measure has evolved into something much more significant: a comprehensive drug safety program that has fundamentally changed how the institute protects its patients.
Over the past five years, the pharmacovigilance efforts of the ICPV and PFU have expanded from pandemic response to encompass all patients with heart and kidney diseases while continuing to improve medication safety by identifying, notifying, evaluating, and reporting adverse drug reactions (ADR) observed in patients. The improvements the centre has achieved are encouraging and demonstrate that systematic drug safety monitoring can deliver tangible benefits for patient care.
All ADRs are reported by the PFU to the ICPV, which ensures the quality and accuracy of each submission through a number of procedures. Aside from ensuring that each report is submitted in the proper format to VigiFlow, the ICPV also conducts causality assessment using the WHO algorithm, performs additional follow-up to monitor outcomes for severe cases, and runs a clinical assessment of ADR reports by reviewing the patient's medical record and the clinical development of the ADR.
Such activities have resulted in marked improvements in ADR reporting from the institute. From the initial creation of the ICPV in May 2020, a total of 2342 notifications have been received, with 1876 submitted to VigiFlow after report validation and duplicate detection (see Figure).
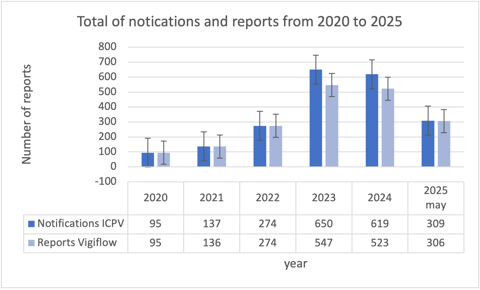
Aside from an increase in the number of ADR reports submitted to VigiFlow, the overall average of incidence reports per year has steadily increased almost threefold from 5.21% in 2020, when the ICPV was created, to 14.38% in 2025 (see Table).
Table: Average incidence of reports per year
| Year | Average incidence of reports per year |
| 2020 | 5.21% |
| 2021 | 3.99% |
| 2022 | 5.05% |
| 2023 | 9.20% |
| 2024 | 9.60% |
| 2025 | 14.38% |
This threefold increase in submitted ADR reports is largely attributed to specific pharmacovigilance initiatives: pharmacovigilance investigations of COVID-19 treatments in 2021, an active pharmacovigilance study of the use of anticoagulant treatment in 2022, and a focus on improving reporting procedures in the ICPV in late 2024 and early 2025.
Aside from our focus on ADR report submission, the ICPV also conducts additional statistical analysis on all reports submitted to VigiFlow every month to evaluate reporting trends and identify clinically relevant clusters—recurring patterns of the same adverse drug reaction reported across multiple patients. This analysis is presented to the pharmacovigilance committee as well as the heads of the various departments at the National Institute of Cardiology to determine the root cause of the reported ADRs and, when possible, establish and evaluate risk minimisation measures to address them, such as:
Every six months, the ICPV compiles a comprehensive activity report for the National Centre of Pharmacovigilance. These reports document the main issues presented to the pharmacovigilance committee, key decisions taken and how well its risk reduction measures have been implemented over the previous six months. The ICPV also include updates on its broader engagement activities – training workshops for staff, health promotion campaigns, participation in initiatives like #MedSafetyWeek, any new scientific publications from our work, and contributions to professional conferences.
Through the implementation of the ICPV, it has been possible to identify preventable ADRs related to medication errors by creating strategies, together with the PFU, to improve medication safety for patients. The centre's systematic approach has yielded measurable improvements in safety surveillance and actionable pharmacovigilance interventions, directly enhancing patient safety outcomes.
The ICPV's integration of clinical assessment, passive and active surveillance, and implementation of risk minimisation measures represents a valuable framework for other healthcare institutions seeking to strengthen their pharmacovigilance capabilities and achieve tangible patient safety improvements.
This article is a follow-up to a previous Uppsala Reports article on the establishment of the ICPV at Instituto Nacional de Cardiología Ignacio Chávez. One of the authors, José Antonio Maza Larrea, now at Uppsala Monitoring Centre, has written about the work conducted during his previous employment at the institute.
Read More:
Two-thirds of pharmacists in Nigeria witness weekly cough syrup abuse, yet poor reporting systems and unclear guidelines prevent effective intervention, leaving its youth at risk.
03 December 2025
More than 200 participants gathered in Mérida, Mexico, to discuss regional cooperation and emerging technologies in medicines safety across Latin America.
09 October 2025
The annual #MedSafetyWeek campaign reminds us that medicines work best when they're safe. In Aligarh, healthcare workers came together to make that message a living reality.
19 November 2025