
COVID-19 vaccine safety surveillance has had a welcome technology boost, with a new release of VigiFlow making it easier for immunisation programmes to monitor vaccine safety data and bring safety issues to the attention of national regulatory authorities (NRAs).
As vaccines are approved and administered to patients on an unprecedented scale, efficient, consistent reporting and analysis of suspected adverse events following immunisation (AEFI) will be vital in protecting the public.
The new functionality makes AEFI more transparent to NRAs and encourages countries to share their data with the WHO Programme for International Drug Monitoring through the WHO global database – VigiBase – as emphasised in WHO’s COVID-19 Vaccines: Safety surveillance manual.
Product manager Paulina Nakielny says UMC has been working closely with the pharmacovigilance team at WHO to make sure the AEFI expansion meets national centre needs in view of the new WHO guidelines around vaccine surveillance and safety monitoring.
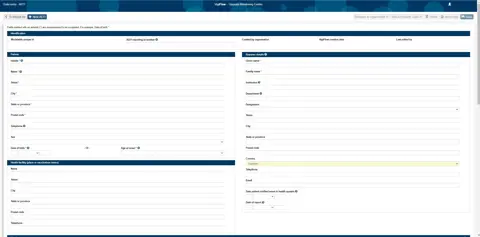
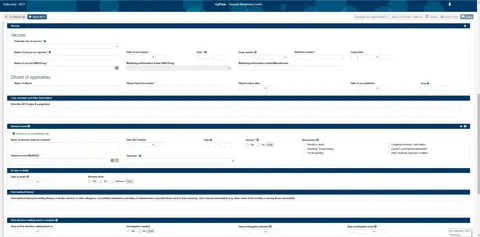
The new functionality includes improved support for entering AEFI reports in VigiFlow in a format that is more recognisable to national immunisation programmes. An AEFI-specific Excel output has also been created to support data analysis. All the improvements are translated into the VigiFlow languages.
“This simplifies the process of capturing vaccine-related information and makes it easier to work with as cases can be extracted into an AEFI line listing Excel sheet for statistical follow-up and analysis,” Nakielny says.
Up until now VigiFlow has largely been used to collect and analyse reports of suspected adverse effects of medicines. The new development will expand the capacity of VigiFlow to support the case management and analyses of AEFI. Analyses performed by AEFI centres, focusing on identifying potential vaccine safety issues such as immunisation errors and the performance of AEFI surveillance systems, can also be performed in VigiFlow.
Rebecca Chandler, senior pharmacovigilance expert within the Pharmacovigilance Product Management team, says as well as enhancing the collaboration between NRAs and immunisation programmes, the integration of AEFI reporting into VigiFlow could see more countries contributing vaccine reports to the WHO programme for International Drug Monitoring – making it easier to identify potential safety concerns.
“The challenge of COVID-19 is a global one in which what happens in one country has the potential to affect many others in different parts of the world,” she says. “Never before has global sharing of safety data been so important.”
To help new users get started a quick guide and a series of short videos explaining how to enter and analyse AEFI data in VigiFlow are available on the UMC website. Pharmacovigilance product support staff have also held webinars focusing on the new data entry form, WHODrug coding of COVID-19 vaccines, and AEFI line listings, in English, French and Spanish.
Hosted by UMC and Morocco's Rabat Collaborating Centre, the webinars have been well attended. “We have had a really enthusiastic response with 200 stakeholders in 42 countries taking part so far,” says pharmacovigilance officer Therese Lundin, one of the moderators for the Q&As.
Now that the technology is available, the next step will be to focus on the practicalities of implementation and assist users in adapting their own processes.
Want to know more?
Visit the UMC website or email vigibase@who-umc.org





