
In its bid to become a fully-fledged member of the International Council for Harmonisation (ICH), in 2019 the Colombian National Institute for Medicines and Food Surveillance (INVIMA) replaced its in-house adverse event reporting system with VigiFlow. VigiFlow is an ICH-compliant national software application for processing and managing adverse event reports from all reporters – patients, healthcare providers, and, crucially, marketing authorisation holders (MAHs).
About the ICH
The International Council for Harmonisation is responsible for developing ICH E2B(R3) - the international standard for exchanging information on the suspected side effects of medicines and vaccines. To become a member of ICH, countries must have systems that are compliant with this format.
ImplementING VigiFlow now allowed INVIMA to effectively send individual case safety reports (ICSRs) in the correct format to the WHO global database, VigiBase. However, its safety reporting process was still hampered by the fact that MAHs, a valuable source of post-marketing safety data, do not have ICH-compliant databases. As a result, the Colombian national centre had to enter any ICSRs received from MAHs (which constitute half or more of the ICSRs received by regulatory authorities) into VigiFlow manually, a task that further strained already limited pharmacovigilance human resources at the national centre.
“Before VigiFlow and Industry eReporting, we had to enter safety reports manually from emails received from industry into our own system which crashed often, and was not ICH E2B compliant,” says Maria Victoria Urrea Duque, the pharmacovigilance officer at INVIMA.
This is where industry eReporting can help. Industry eReporting is an add-on to VigiFlow that allows companies to submit post-marketing and clinical trial safety data directly to a country’s national database in the ICH E2B format. It also incorporates the Medical Dictionary for Regulatory Activities (MedDRA) for coding of medical and regulatory terminology, as well as WHODrug for coding of medicinal products.
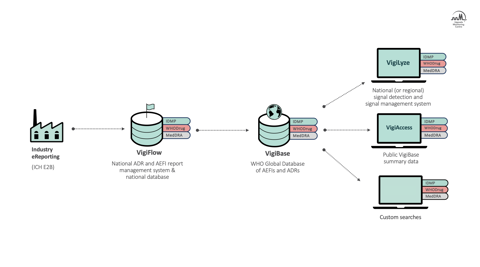
By making the transfer of safety data more efficient between industry and national centres, staff can start working with reports and identify potential safety risks and obstacles to regulatory approval faster. INVIMA began implementing Industry eReporting in the second half of 2021, a process which, according to Duque, has “generated confidence in the companies responsible for updating their pharmacovigilance processes to these new local regulations, and strengthened the skills required to effectively use the tool”.
THE use of industry eReporting has already had a positive impact on the quantity and quality of reports from MAHs and has also helped several authorities comply with their international commitments to acquire ICH standards in their pharmacovigilance activities. As this ereporting tool was developed (and is continuously updated) by the pharmacovigilance products teams at Uppsala Monitoring Centre with feedback from many national and transnational companies from different countries, the tool has been well received by industry and regulators, an outcome that Duque has also observed at INVIMA:
“The implementation of Industry eReporting into our pharmacovigilance system has resulted in a total of 264 pharmaceutical companies with access to the interface and has been well accepted by the industry here in Colombia. As a result, more than 110,000 reports have been received over the last two years, which is over two times more than the number of reports we were receiving before we started using VigiFlow. These reports are greatly improved in quality with the use of the inbuilt MedDRA and WHODrug standards and are transmitted much faster and far more efficiently than before implementation of the tool.”
Read more:





