
Federica Santoro
Communications Officer, UMC

Med Safety Week 2018
Uppsala Monitoring Centre has launched the third annual social media campaign to promote the importance of reporting suspected side effects from medicines.
Taking place from 19-23 November, the campaign forms part of an awareness week involving 32 medicines regulators in the EU, Latin America, Australasia and the Middle East. Regulators will jointly focus on raising reporting numbers for suspected side effects in infants and children, and during pregnancy, including when breastfeeding.
Regulators rely on the reporting of suspected side effects – also known as adverse drug reactions – to make sure medicines on the market are acceptably safe. Unfortunately, all reporting systems suffer from under reporting – this is why the campaign is important to both raise awareness and help strengthen the system. The 2017 campaign reached 2,3 million people on social media, and resulted in an increase of 11% in reporting of adverse drug reactions.
The campaign is being taken forward through the Heads of Medicines Agencies Working Group for Communications Professionals and the International Coalition of Medicines Regulatory Authorities. A number of other organisations – including the European Medicines Agency, the European Commission and patient organisations such as EURORDIS – have also pledged their support. This year, the United Nations’ Universal Children’s Day falls within the awareness week, and both campaigns seek to promote international togetherness and improve children's welfare.
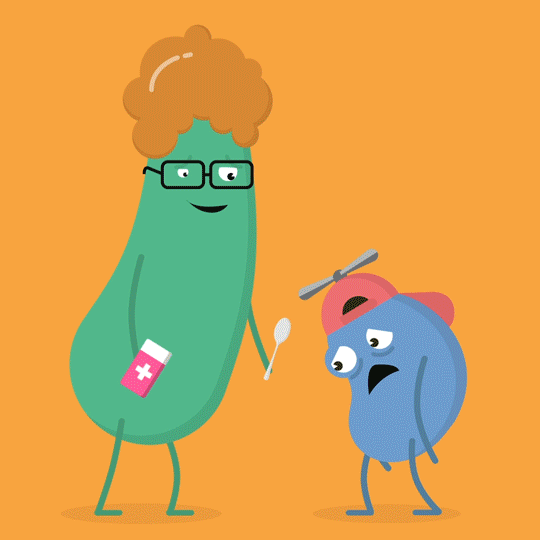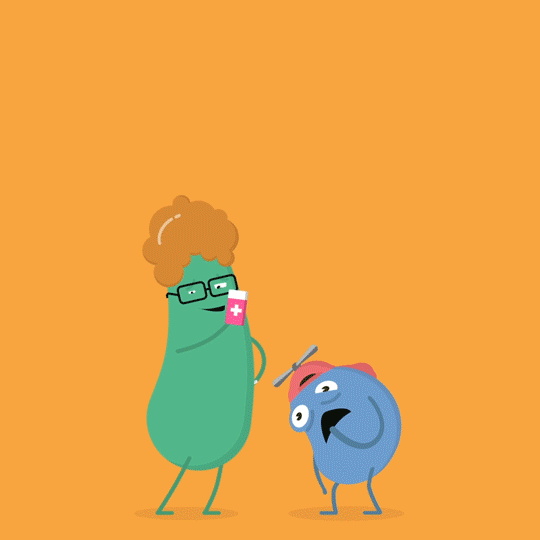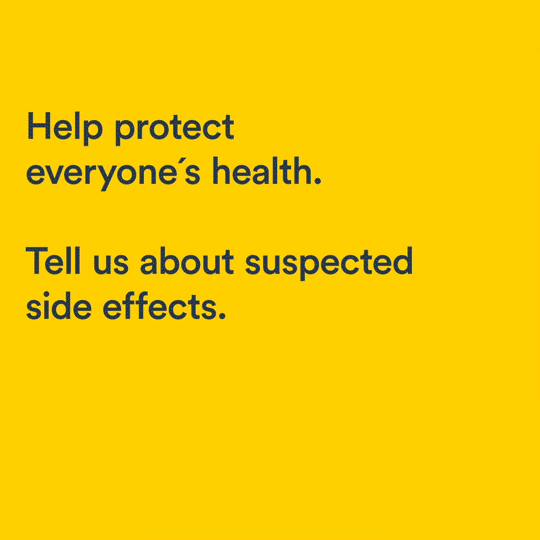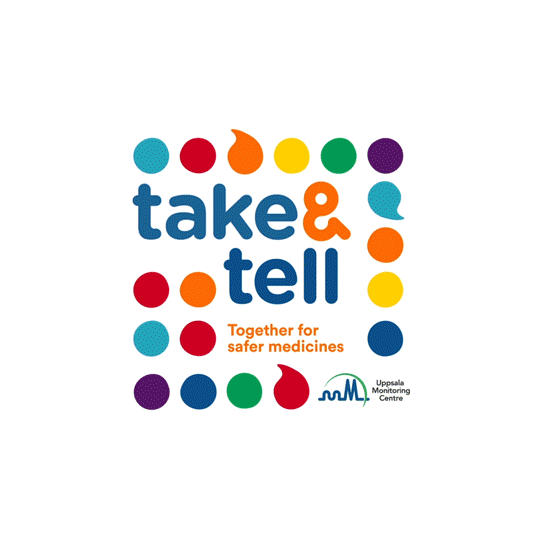
Campaign materials include a light-hearted and amusing series of animations produced by UMC, featuring funny cartoon characters whose unfortunate misuse of medicines leads to comical calamities. The short animations have been adapted for use in the 32 countries. Each country-specific version features text in the local language, the logo of the medicine regulatory authority and a link to the national reporting system.
Besides making the animations available to the countries, UMC will also run the campaign on its social media channels. Connect to us with the hashtag #MedSafetyWeek, or follow us on social media:
You can also follow any of the 32 participating medicines agencies:
Australia: @TGAgovau
Belgium: @FAGG_be (Dutch); @AFMPS_BE (French)
Bulgaria: www.bda.bg
Colombia: @invimacolombia
Croatia: www.halmed.hr
Czech Republic: @SUKLcz
Denmark: @LMSTdk (Danish);@DKMA_dk (English)
Ecuador: @Arcsa_Ec
El Salvador: @MedicamentosSV
Estonia: @ravimiamet
Finland: @Fimea
France: @ansm
Germany: @bfarm_de
Greece: www.eof.gr
Hungary: @ogyei.gov.hu
Iceland: @lyfjastofnun
Iraq: @MOH.GOV.IQ
Ireland: @TheHPRA
Italy: @Aifa_ufficiale
Jordan: @Jordan_FDA
Latvia: @ZVALatvija
Lithuania: @vvkt.lt
Malta: @medicinesmalta
Mexico: @COFEPRIS
New Zealand: @minhealthnz
Poland: @URPLWMiPB
Portugal: @INFARMED_IP
Romania: @anmdm.ro
Slovak Republic: @sukl_sr
Slovenia: @AgencijaJAZMP
Sweden: @LV_MPA
United Kingdom: @MHRAgovuk
AI is already reshaping drug safety. Niklas Norén explores how to harness its power responsibly in this two-part episode.
26 November 2025
AI is already reshaping drug safety. Niklas Norén explores how to harness its power responsibly in this two-part episode.
22 October 2025
Discover how researchers are addressing crucial challenges in pregnancy-related pharmacovigilance to better protect both mothers and their unborn children.
03 April 2025