
Alemayehu (Alex) Duga
PAVIA PV coordinator- Eswatini

Photo: iStock
Eswatini may be a small country, but its National Pharmacovigilance Centre has big ambitions that, with support from the PAVIA project, are quickly translating into achievements.
Integrating pharmacovigilance as an essential component of public health programmes is crucial for patient safety. To this end, the Ministry of Health (MOH) in Eswatini took the initiative to establish a national pharmacovigilance unit in 2009. It started with a spontaneous reporting system, as it represents one of the most important sources of information for assessing the safety and quality of drugs on the market. Much has happened since then.
In 2015, the introduction of new TB medicines prompted the introduction of an active sentinel site reporting system for drug-resistant TB and HIV medicines. Then, in 2018, through the pharmacovigilance unit, Eswatini MOH implemented Pharmacovigilance in Africa (PAVIA), a project aimed at strengthening national PV systems in four African countries by building and strengthening collaborations and the ties of all the major pharmacovigilance players to ensure effectiveness and sustainability. PAVIA is a consortium funded by the European Developing Countries Clinical Trials Partnership, which aims to strengthen pharmacovigilance in Ethiopia, Nigeria, Eswatini, and Tanzania. To achieve this aim, collaborative support will be harnessed across several institutions in Europe and Africa.
The introduction of the PAVIA project has helped in streamlining the medicine safety surveillance activities in the context of introducing new drugs for multidrug-resistant tuberculosis. In 2018, PAVIA and the pharmacovigilance unit conducted a baseline assessment on the pharmacovigilance status in Eswatini. This work informed the development of a project roadmap and work plan, leading to multiple achievements, not the least of which was the elevation of the pharmacovigilance unit into a National Pharmacovigilance Centre (NPC), as well as recruitment and training of NPC staff, and further training and capacity building for healthcare workers at different levels.
Currently, the NPC consists of five well-trained staff members (four pharmacists and one pharmacy technician). This professional capacity has enhanced the capturing of reports into VigiFlow, regular analysis and publication of a medicine safety newsletter, and the first ever signal detection activity at the centre.
The Eswatini NPC adapted the PAVIA “triangle model”, which involves formalised and intense collaboration between trios of pharmacovigilance stakeholders to strengthen relevant activities in the country. Following this model, the NPC works with multiple key stakeholders and public health programmes to assist in introducing new medicines and monitoring medicines safety.
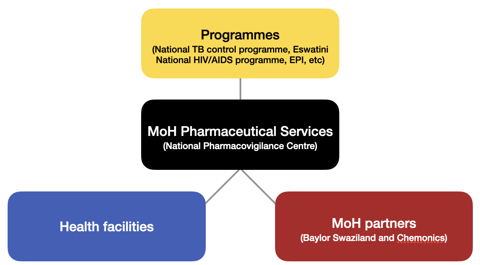
The NPC is also involved in the development of various public health programme guidelines, SOPs and safety information bulletins. Some of its achievements include the successful roll-out of the dolutegravir-containing HIV treatment regimen and implementation of active drug safety and monitoring (aDSM) for multiple drug-resistant tuberculosis (MDR-TB) treatment. To achieve these, the centre employed a number of strategies, including reviewing and editing of reporting tools, training of healthcare workers, and providing onsite supportive and mentorship visits. As of January 2022, the centre has submitted more than 1170 individual case safety reports to VigiBase, which is a significant increase since the introduction of PAVIA project.
Several features appear to have contributed to the increase in both reporting rate and report quality. In particular, the NPC’s visibility has enabled a close collegial relationship with reporters, and each reporter has received an acknowledgement letter from the NPC. Also, a contributing factor to the increasing reporting rate is the programmes that have run side-by-side with NPC.
Looking into the future, the NPC sees major factors that could influence further improvement, namely the decentralised implementation of VigiFlow into facilities and public health programmes to increase adverse event reporting, and recruitment of more NPC staff.
Training will also be critical. Following a master-trainer strategy, NPC staff have been trained in data collection and management, signal detection and causality assessment, risk-benefit evaluation, and decision making. The trainings were provided through the PAVIA project in collaboration with the Netherlands Pharmacovigilance Centre Lareb. The main objective of this training is to empower staff to efficiently undertake pharmacovigilance activities at a central level, provide training to colleagues at facility level, and increase pharmacovigilance awareness across the country. NPC staff and relevant focal people within public health programmes have benefited from this training plan.

One of NPC’s strategies is to create pharmacovigilance awareness in pre-service training through short sessions incorporating a pharmacovigilance curriculum as a module of the regular undergraduate course. Following this strategy, NPC, in collaboration with Southern African Nazarene University (SANU), provided training to graduating students to increase their awareness around and implementation of pharmacovigilance activities as they enter the field. The aims of the training were to ensure learners are able to identify the key pharmacovigilance concepts, basic terms, and definitions; describe how to implement and manage aDSM within the facility; understand how to detect, manage, and report adverse events; record adverse events and ensure quality of data records; and understand key concepts of causality assessment and signal detection.

Another strategy was to provide PAVIA “blended training” to healthcare professionals in the health facilities involved in MDR-TB treatment and staff at the national TB programmes. This entails delivering a basic pharmacovigilance training through a blended learning approach, combining e-learning with face-to-face interaction, where more in-depth discussions are held. The e-learning modules have been developed on a platform called Schoolrooms, where the participants log in to access the training material. This platform also helps to assess the knowledge of participants before and after the training. The courses consist of two components, one focusing on generic pharmacovigilance aspects, another on specifics for MDR-TB treatment. This training is delivered through the University of Verona, a PAVIA project partner. To date, 34 participants have received a train-the-trainer course, building capacity to extend training to other healthcare workers in the country.



Beyond training, the NPC has also made achievements in other areas. In September 2021, NPC collaborated with Southern African Nazarene University (SANU) pharmacy students and Raleigh Fitkin Memorial Hospital community and patients to celebrate international patient safety event, World Patient Safety Day. The various activities and resources included presentations, one-on-one interactions with patients, leaflets, and poetry. Key pharmacovigilance stakeholders, including medicine importers, private pharmacies, and academia took part in the event.

One of NPC’s most significant achievements to date came on 6 December 2021, with the launch of the Eswatini National Pharmacovigilance Policy and Implementation Framework by the Honourable Minister of Commerce Industry and Trade, Manqoba Khumalo, representing the Honourable Minister of Health at a hybrid-format event in Mbabane. The policy document was developed under the NPC’s leadership within the Medicines Regulatory Unit of the Ministry of Health. PAVIA Work Package (WP 2) leaders and various partners also provided technical and financial support.
The event was attended by key officials from the Ministry of Health and the Ministry of Commerce, Industry and Trade, along with representatives from the PAVIA Coordination Office, public health programmes, country directors of various MoH partners, Eswatini Health and Human Research Review Board, World Health Organization, Regional Health Management Team, healthcare workers, and the Eswatini Pharmaceutical Association, among others.

Addressing the audience, Minister Khumalo, on behalf of the health minister said, “I would like to emphasise that the Ministry is fully committed to the implementation of this policy document".
"I would also like to call upon all of you, the concerned stakeholders to actively do your part by supporting the various aspirations which the Ministry seeks to achieve through this policy. Make sure that you not only get a copy of this document, but also contribute your part by supporting its implementation.”
Two-thirds of pharmacists in Nigeria witness weekly cough syrup abuse, yet poor reporting systems and unclear guidelines prevent effective intervention, leaving its youth at risk.
03 December 2025
The annual #MedSafetyWeek campaign reminds us that medicines work best when they're safe. In Aligarh, healthcare workers came together to make that message a living reality.
19 November 2025
Since 2020, the ICPV has worked to improve drug safety reporting at Instituto Nacional de Cardiología Ignacio Chávez, with promising outcomes.
16 October 2025