
Helena Sköld
@NennePennen
VigiBase Manager, UMC

Photo: iStock
After decades of slow but steady growth, VigiBase – WHO’s global database of reported potential side effects of medicinal products – has recently entered a new phase of accelerating expansion, driven by a range of converging forces.
In the early days of the World Health Organization’s Programme for International Drug Monitoring (WHO PIDM), reports of suspected adverse drug reactions were sent to Uppsala Monitoring Centre (UMC) as paper copies to be manually entered into VigiBase. Today, if each report in VigiBase were printed onto a single sheet of paper, and all the reports were stacked on top of each other, the stack would reach more than three times the height of the world’s tallest building – Dubai’s Burj Khalifa.
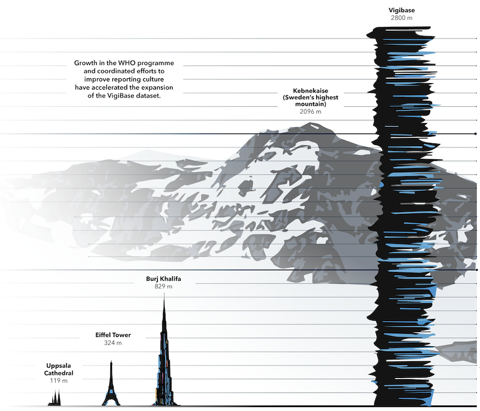
VigiBase is the largest database of its kind in the world, with records of reported potential side effects of medicinal products submitted by member countries of the WHO PIDM dating back to 1968. Until about a decade ago, VigiBase growth was relatively slow. But an increasing awareness of the importance of global pharmacovigilance – reflected in changes to national and regional law, regulation, and practice – have led to much higher levels of both reporting and sharing of data. On 25 February 2021, the number of reports in VigiBase passed 25 million, then continued to surge past 26 million by 12 May. Only four months later another 2 million reports have been entered.
The most recent phase of rapid growth can, to a large extent, be explained by the wave of reports on COVID-19 vaccines, a phenomenon that has been widely encouraged to help support the safe rollout of the new vaccines by gathering data about their safety in as much detail and as quickly as possible. [For more on the value and significance of this new data, see the Q&A that follows this article.]
This has led to a period of intense work focus across most areas within UMC. For example, UMC’s terminology specialists have dedicated considerable resources into covering all the variants of COVID-19 vaccines in WHODrug and manually trying to find more specific coding options for the incoming reports so that analysis can take place at appropriate levels. Our research teams work relentlessly to analyse and assess the data and provide monthly descriptive reports to our VigiLyze users.
At the same time, our product development teams are improving the possibilities for both data capture and analysis in VigiFlow and VigiLyze, and we get more and more opportunities to share our efforts with the world through presentations in different settings. For example, UMC has presented these analyses in work with the Council for International Organizations of Medical Sciences (CIOMS), a collaboration with CIOMS and the Centre of Regulatory Excellence, and training with the International Society of Pharmacovigilance. There are also similar activities being planned with other regional and national organisations.
“Another positive trend is the contribution to VigiBase from low- and middle-income countries, which has increased markedly over the past 10 years.”
In more general terms, contributions to VigiBase are still dominated by US reports, which comprise 45% of the data,with the European Union contributing another 20%. The proportion of reports from Asia, however, is rising strongly,with China, Korea, and India making larger shares of the total and annual contributions.
Another positive trend is the contribution to VigiBase from low- and middle-income countries, which has increased markedly over the past 10 years from around 5% to more than 15%.
With improved reporting frequencies during the pandemic from big contributors such as the US and China, timeliness of reporting is improving significantly. This factor is also aided by countries implementing the VigiBase API, which enables more manageable continuous reporting rather than large periodic batches.
Uppsala Reports caught up with our colleague, VigiBase Manager Helena Sköld, for a quick look at what this remarkable VigiBase growth means.
Pharmacovigilance is a data-dependent science, and we will always struggle with underreporting, so this growth is invaluable. At UMC, the methodological developments that our data science researchers have pioneered for quantitative signal detection, combined with increasingly vast datasets, are making pharmacovigilance more powerful and sophisticated than ever. More and more national agencies are also adopting UMC’s quantitative methodologies and, again, this is probably also contributing to the growth in record sharing.
For many years, the number of countries participating in the WHO programme was low, but that has changed, and we now have 148 full programme members sharing reports to VigiBase. In particular, growth among lower- and middle-income countries – many of whom have large populations has been strong. During that time, we have seen a corresponding growth in awareness of the importance of pharmacovigilance for patient safety. We also see in the legislative and regulatory frameworks of more countries a move to strengthen the reporting culture. The methods of sharing reports have also improved greatly over years, and the investments UMC has made in developing tools and processes – not to mention pharmacovigilance training and capacity building – are really paying off in terms of volume and quality of data.
If some national agencies are finding it difficult to cope with the current avalanche of data, I would suggest they should be looking to tools like VigiLyze to improve their quantitative analytic abilities. It’s also very important to remember that these quantitative signal detection methodologies are still only used to develop hypotheses. Any suspected signals must then be investigated with in-depth assessments of case data, scientific literature, and other data sources.
No, quite the contrary. Because this is the biggest mass vaccination programme in history, it makes sense that there would be a large number of reports anyway. But also, because these are new vaccines and they are so important to stopping the pandemic, health authorities at national and international level have been actively encouraging reporting. The amount of data we are receiving, and the timeliness with which we receive it, means that safety monitoring can be carried out at unprecedented scale and speed. In fact, what we are seeing is the safety monitoring systems working extremely well. This gives us a way to monitor rare events that could not be found in clinical trials.
As bad as the pandemic has been, it has at least created unprecedented collaboration between national regulators and national immunisation programmes, especially in lower- and middle-income countries. So, I believe what we are seeing is not a sign of the danger of these new products, but a sign of confidence. It says to me that we have more data about the real-world safety of these products than any other medicinal product before.
South Africa’s pharmacovigilance system has been evolving for over a decade with UMC’s data management system supporting them every step of the way.
02 September 2024
Disproportionality analysis is pharmacovigilance’s signal-detection workhorse. But more reports do not mean better evidence, and crude results can mislead.
05 February 2026
Egypt's swift detection of vincristine-associated necrotising infections demonstrates how effective pharmacovigilance can identify risks and protect patients globally.
08 May 2025